Abstract
Introduction: Low-grade B-cell lymphomas (LGBCL), aside from follicular lymphoma and chronic lymphocytic leukemia/small lymphocytic lymphoma, account for approximately 10% of B-cell non-Hodgkin lymphomas and consist of several subtypes. While a majority of LGBCL cases have an overall favorable prognosis, we have previously shown that cases who have an event (relapse or progression, transformation, or re-treatment) within 24 months of diagnosis (EFS24) have an inferior overall survival (OS) compared to those achieving EFS24 (Tracy et al., AJH 2019;94:658-66). However, the underlying biological characteristics associated with early failure and aggressive disease across LGBCL subtypes are unknown. In this study, we used matched transcriptomic, genomic, and immune profiling data from LGBCL cases, the largest cohort to date, and asked whether there were unique biological phenotypes across different LGBCL subtypes and whether we could identify signatures associated with aggressive LGBCL. Validation of the prognostic utility of this signature was performed on a previously published, independent cohort of 63 pre-treatment LGBCL cases.
Methods: Tumors from 64 newly diagnosed LGBCL patients from the Molecular Epidemiology Resource of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence were included in this study (SMZL (n = 48), NMZL (n = 6), LPL (n = 5), B-NOS (n = 3), EMZL (n = 2)). RNA sequencing (RNAseq) data from 61 LGBCL tumors and 5 benign CD19+CD27+ memory B samples was subjected to NMF clustering to define groups. Differential expression and pathway analysis were used to identify biological characteristics of each cluster. CIBERSORT was used to identify immune cells in the tumor microenvironment. Whole exome sequencing (WES) was performed on 61 tumor-normal pairs. Singscore was used to assign a single score per patient representing gene expression of the survival-associated transcriptomic signature identified in this study.
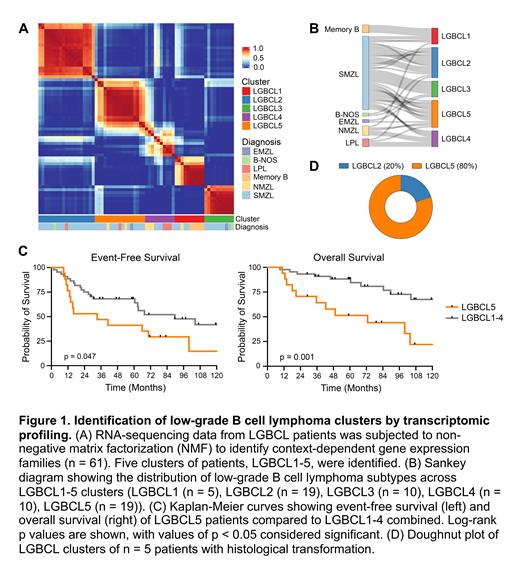
Results: NMF analysis of RNAseq data identified 5 clusters of patients, denoted LGBCL1-5 (Fig 1A). Patients from the same diagnostic subtype did not exclusively cluster together, with all LGBCL clusters comprised of patients from multiple subtypes (Fig 1B). Exploring the association between patient cluster and outcome, we observed significantly inferior event-free survival (EFS) (HR 2.24; 95% CI 1.01-4.98) and overall survival (OS) (HR 5.59; 95% CI 2.00-15.63) in LGBCL5 patients compared to LGBCL1-4 (Fig 1C). In addition, 80% of the transformation cases in our cohort were classified as LGBCL5 (Fig 1D). Differential expression and pathway analysis showed distinct processes significantly upregulated in each cluster (FDR < 0.05), with LGBCL5 demonstrating enrichment of cell cycle and mitosis pathways. CIBERSORT identified increased immune cell content in LGBCL3 and LGBCL5 compared to other clusters, with high frequencies of mast cells in both (p = 0.0002), increased CD8 T cells in LGBCL3 (p < 0.0001), and increased T follicular helper cells in LGBCL5 (p = 0.004). WES identified previously reported alterations in NOTCH, NFkB, and chromatin remodeling pathways and novel variants in LGBCL, including mutations in HNRNPK, CLTC, HLA-A, HLA-B and HLA-C. Assessment of alterations by cluster showed significant enrichment of TNFAIP3 (OR 5.54; 95% CI 1.20-28.14) and BCL2 alterations (OR 5.49; 95% CI 1.07-32.02) in LGBCL5 cluster. Finally, we identified a cell cycle-related transcriptomic signature of 108 genes upregulated in LGBCL5 and EFS24 failure cases. Cases with high expression of this signature showed significantly inferior EFS (HR 14.25; 95% CI 4.90-41.38) and OS (HR 7.82; 95% CI 2.40-25.44) compared to cases with low expression in our discovery cohort. This observation was reproduced in an independent validation cohort, where patients with high expression of this signature demonstrated significantly inferior EFS (HR 5.70; 95% CI 1.49-21.79) and OS (HR 10.07; 95% CI 2.00-50.61).
Conclusions: In this study, we are the first to define mechanisms of pathogenesis in LGBCL with shared transcriptomic, genomic, and immune profiles present across LGBCL subtypes. We then further defined a gene expression signature associated with inferior patient outcome, with application of this signature to an independent validation cohort demonstrating proof of concept and utility of this signature as a prognostic marker in LGBCL patients.
Maurer: Morphosys: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Nanostring: Research Funding. Paludo: Karyopharm: Research Funding. Habermann: Tess Therapeutics: Other: Data Monitoring Committee; Morphosys: Other: Scientific Advisory Board; Incyte: Other: Scientific Advisory Board; Seagen: Other: Data Monitoring Committee; Loxo Oncology: Other: Scientific Advisory Board; Eli Lilly & Co.,: Other: Scientific Advisor. Link: MEI: Consultancy; Genentech/Roche: Consultancy, Research Funding; Novartis, Jannsen: Research Funding. Rimsza: NanoString Technologies: Other: Fee-for-service contract. Ansell: Bristol Myers Squibb, ADC Therapeutics, Seattle Genetics, Regeneron, Affimed, AI Therapeutics, Pfizer, Trillium and Takeda: Research Funding. Cerhan: Genentech: Research Funding; Regeneron Genetics Center: Other: Research Collaboration; Celgene/BMS: Other: Connect Lymphoma Scientific Steering Committee, Research Funding; NanoString: Research Funding. Novak: Celgene/BMS: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal